*G32 Local Application in Patients undergoing Orthodontic treatment for Malocclusion
**Mrs. Shivaratna C. Savadi, M.D.S., Dr. Reena Ranjit Kumar, B.D.S.
***Mrs. K. V. Vijaya, B.D.S., D.(Orthodontics)
Introduction
The main objective of orthodontic treatment is to restore the acceptable function of the stomatognathic system by the correction of dental irregularity. It also gives a better personal appearance. A survey of the literature shows that about 65% of Indian population need orthodontic treatment. Considering the socio-economic criteria, the use of removable appliances in larger number of cases is justified. Thus, in India more patients are being treated with removable appliances rather than with fixed appliances.
A number of questions arise while undertaking orthodontic treatment. The condition of the gums and alveolar bone may not be as good as the orthodontist’s wishes. Should the orthodontist stay away for fear Of making the condition worse? What is the advisability and prognosis for orthodontic treatment in bad periodontal condition? Further some authors have shown that pathological changes occur in the gingiva following the use of orthodontic appliances.
Zacchrisson & Zacchrisson, Skillen and others have confirmed that in spite of good oral hygiene, the majority of the patients undergoing periodontic treatment usually develop generalised moderate gingivitis within one or two months after the placement of orthodontic appliances. This effect will be exaggerated in cases of poor oral hygiene and poor periodontal condition. Hence every effort should be made to maintain optimum periodontal health before, during and after orthodontic treatment.
If gingival inflammation is allowed to go unchecked or is neglected, it may cause degeneration of periodontal ligament fibres and impair their ability to transmit external forces to the bone. This dissipates orthodontic forces and delays the desired bone response and tooth movement in orthodontic treatment.
To prevent these problems as far as possible, a well planned home-care programme including tooth brushing and a good gum massage every day is essential. For this purpose, G32 an Ayurvedic drug was selected for clinical trial.
Orthodontic tooth movement and Gingival inflammation
The force exerted by the orthodontic appliance is greater than that produced during physiologic tooth movement. Hence the periodontium is subjected to a greater stress during orthodontic tooth movement. The presence of periodontal disease is not a contra-indication for orthodontic treatment. Patients undergoing orthodontic treatment are more susceptible to deepening of the gingival sulcus and subsequent bone resorption. Orthodontic tooth movement which stimulates trauma from occlusion is responsible for causing destruction of alveolar bone, periodontal ligamentum and even root surface. Zacchrisson & Zacchrisson in a longitudinal study in orthodontic patients treated with fixed appliances, found that gingivitis developed inspite of the use of flouride mouth washes. Intra-proximal areas were more affected than buccal areas. Epithelial attachment migrated by 1. mm every year in patients not given instructions on maintaing good oral hygiene, while it was only 0.08 mm in patients who were given instructions.
G32
G32 is an ethical Ayurvedic research product and is available in easily crushable tablet form to be crushed and used for application to the gingiva, teeth and other tissues in the month. It is described to have astringent, antiseptic, anti-inflammatory, antacid, anodyne, styptic, deodorant, aromatic, cooling and healing properties. It improves oral hygiene in health and sickness. It can be used as local application, gum massage, rinse, gargle and dentifrice.
Studies conducted by Agarwal, Ajgaonkar, Ali, Banerjee, Baskar, Bhatnagar, Boghani, Goel, Kamat, Mohanty, Nagesh, Prasad, Radhakrishnan, Rajasekhar, Rao, Savanur, Shah, Srivastava, Vachrajani, Varghese, Varma, Yadav show the efficacy of G32 in various periodontal conditions. They found G32 to be an effective and a safe drug in the treatment of Gingivitis, acute or chronic, localised or generalised and even when associated with painful teeth, bleeding and enlarged gums. In the light of these, in this trail, an attempt is made to evaluate the efficacy of G32 in orthodontic cases with poor periodontal condition.
G32 is for local application and this has an advantage over mouth washes now routinely used. Also, nowadays, the people and the medical profession are taking increased interest in Ayurveda, our national heritage which has stood the test of time since centuries.
G32 Composition
Each tablet of G32 contains: —
Baku! (Mimsops Elangi) 80 mg., Chok (Calcium Carbonate) 75 mg., Katho (Acacia catechu) 40 mg., Laving (Myrtus caryophyllus) 20 mg., Chikani Sopari (Areca catechu) 20 mg., Fatakadi (Alumen) 20mg., Mayafal (Quercus Infectoria) 20 mg.,Elaichi (Elettaria Cardomomum) 10 mg., Sonageru (Silicate of Alumina & Iron Oxide) 10 mg., Jiru (Carum Carui) 10 mg., Majith (Rubia cordifolia) 10 mg., Pashanbhed (Saxifruga Ligulata) 10 mg., Vavding (Embelia Ribes) 10 mg., Pipalani Lakh (Ficus Religiosa) 10 mg., Samudrafin (Os sapiae) 10 mg., Vajradanti (Barleria Prionitis) 10 mg., Taj (Cinnamomum cassia) 5 mg., Mari (Piper Nigrum) 5 mg., Sajikhar (Sodium carbonate impura) 5 mg., Kulinjan Alpinia Chinenesis) 5 mg., Pipar (Pipar Longum) 5 mg., Kapur (Camphora officinarum) 5 mg. and Kuth (Uncaria Gambier) 5 mg.
Method and Materials
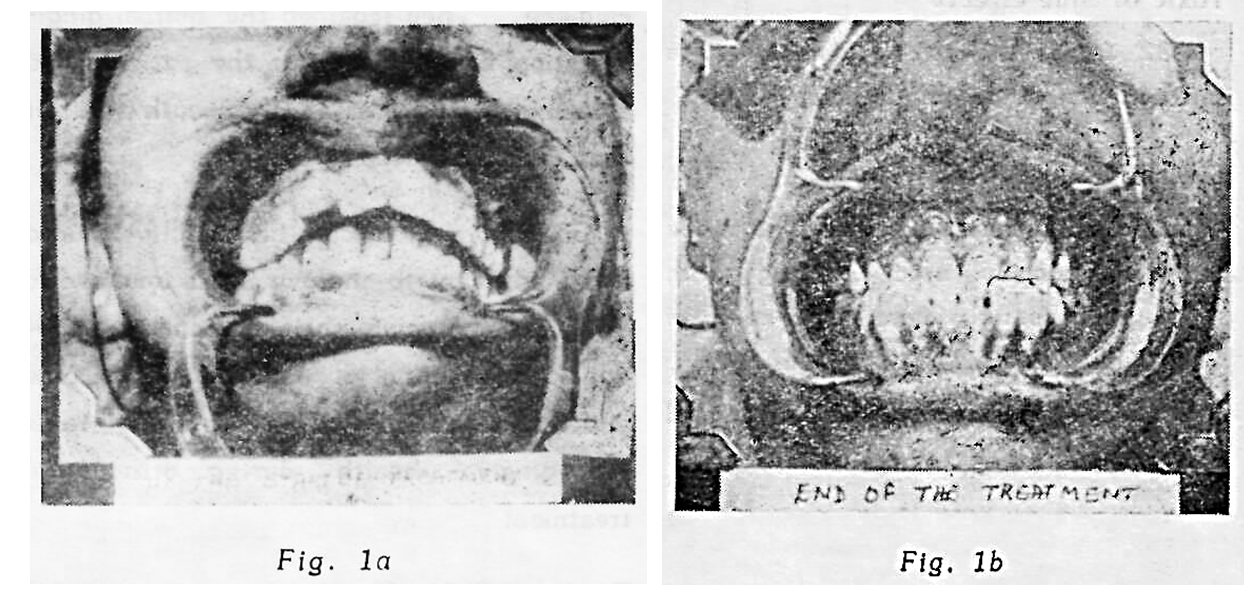
In each case Study Model, Model Analysis and Intta-oral radiographs were done. Pretreatment photagraphs cencentrating on gingiva were also taken.
There were 15 subjects in the present study who were aged between 17-30 years. Amongst them 11 were females and 4 were males. Intra-oral peri-apical X-rays showed good bony support, the bone loss not exceeding cervical 1,3.
Oral Hygiene instructions were given two weeks prior to orthodontic treatment. The patients were instructed to maintain good oral hycjiene by proper and adequate tooth brushing.
These 15 cases were divided into two groups. The First Group consisted of 8 patients and the Second Group consisted 7 patients. The First Group was started with G32 application along with orthodontic treatment. The Second Group was given orthodontic treatment, but G32 application was not started immediately. But, after 4-5 months when the patients showed signs of gingival enlargement, they were advised to use G32 application. Patients were examined once a month for recording GII and OHI. The First Group used G32 application for ten months and the Second Group used it for 5 months of the second half of the ten-month period of clinical trail This study was done at the Bangalore Dental College, Bangalore during 1982-84.
Local Application G32
G32 is available in an easily crushable tablet form. First the patient was asked to gargle with luke-warm water.The patient was advised to crush G32 tablet to powder with a little pressure. The powder was to be applied over the gums and then gently massaged for a few minutes if not painful. The patient was asked to wait for 10-15 minutes for the drug to act and then gargle with luke-warm water. This was to be done once in the morning and once at bed-time. Powder of two G32 tablets was to be used for each application.
Evaluation of results :
Gingival Inflammation Index (GII) & Oral Hygiene Index (OHI)
Regular recording of GII & OHI was made once a month for ten months. The criteria for GII was based on Ramfjords gingival component index and OHI Index was based on OHI-Simplified criteria of Green & Vermillion.
Results of Group I
Gingival Inflammation Index (GII)
In Group 1, G32 application was used along with orthodontic treatment from the start. It was continued for ten months. In the first month Gil was 0,93 mean value of all the cases. It gradually decreased from month to month and at the end of the trail period of ten months, GII was 0.02 (mean). (Table I)
Results of Group II
Gingival Inflammation Index (GII)
In Group II, G32 application was not used along with orthodontic treatment from the start. These developed Gingival Inflammation which gradually increased from month to month. The mean Gil was 0.98 in the first month and it reached to 2.0 (mean) at the end of 5 months when G32 was not used. G32 application was used from 6th month. Since then, GII began to decrease from month to month and at the end of five months of treatment (that is, from 6-10 months), Gil was 0.04. (Table II)
Oral Hygiene Index (OHI)
Oral Hygiene Index both in Group I and Group II showed the same pattern of improvement as in Gil, showing that application of
G32 increased OHI and it was far better in Group I where G32 application was done for 10 months, and it was good in Group II when G32 application was begun midway after 5 months.
Table-I
Gingival Inflammation Index (GII)- Group I: N=8
(In Group I G32 application was used for 10 months)
| Case | Months | |||||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1 | 1.0 | 1.0 | 1.1 | 1.0 | 0.82 | 0.82 | 0.5 | 0.13 | 0.13 | 0.0 |
| 2 | 1.1 | 0.82 | 0.5 | 0.5 | 0.5 | 0.16 | 0.1 | 30.0 | 0 0 | 0.0 |
| 3 | 1.1 | 0.82 | 0.82 | 0.5 | 0.16 | 0.13 | 0.0 | 0.0 | 0.0 | 0.0 |
| 4 | 0.82 | 0.82 | 0.82 | 0.5 | 0.16 | 0.16 | 0.13 | 0.0 | 0.0 | 0.0 |
| 5 | 1.0 | 0.82 | 0.82 | 0.5 | 0.13 | 0.13 | 0.13 | 0.0 | 0.0 | 0.0 |
| 6 | 0.82 | 0.82 | 0.82 | 0.5 | 0.16 | 0.13 | 0.13 | 0.13 | 0.0 | 0.0 |
| 7 | 1.1 | 1.1 | 0.82 | 0.82 | 0.5 | 0.5 | 0.16 | 0.13 | 0.13 | 0.0 |
| 8 | 0.5 | 0.5 | 0.82 | 0.5 | 0.5 | 0.5 | 0.16 | 0.16 | 0.16 | 0.16 |
| Mean Value | 0.93 | 0 84 | 0 84 | 0.60 | 0.37 | 0.32 | 0.17 | 0.07 | 0.05 | 0.02 |
Table-11
Gingival Inflammation Index (GII)-Group II: N=7
| (In Group II G32 application was used | from 6-10 | months) | ||||||||
| Case | Months | |||||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 9 | 1.1 | 1.82 | 1.82 | 2.0 | 2.0 | 1.82 | 1.66 | 1.1 | 0.82 | 0.13 |
| 10 | 1.0 | 1.1 | 1.82 | 1 .82 | 2.0 | 1.82 | 1.1 | 1.0 | 0.3 | 0.0 |
| 11 | 1.1 | 1.1 | 1.82 | 2.0 | 2.0 | 1.82 | 1.1 | 0.3 | 0.3 | 0 0 |
| 12 | 1.1 | 1,82 | 1.82 | 1.82 | 2.0 | 1.82 | 1.66 | 0.3 | 0.0 | 0.0 |
| 13 | 1.0 | 1 82 | 2.0 | 2.0 | 2.0 | 1.82 | 1.66 | 0.3 | 0.0 | 0.0 |
| 14 | 1.1 | 1.82 | 2.0 | 2.0 | 2.0 | 1.82 | 0.13 | 0.0 | 0.0 | 0.0 |
| 15 | 0.5 | 0.82 | 1.1 | 1.82 | 2.0 | 1.82 | 0 82 | 0.5 | 0.13 | 0.13 |
| Mean Value | 0.98 | 1.47 | 1.72 | 1.89 | 2.00 | 1.82 | 1.08 | 0.50 | 0.22 | 0.04 |
Final results and Assessment
All the patients with Gingival Inflammation and poor OHI were successfully treated with G32 local application of 5-10 months the longer the use, better being the results.
Discussion
All patients in the present study responded well to G32 application to the gingiva. In Group II, G32 application was started only after five months of orthodontic treatment and this served partially as control Group. During the first five months Gingival Inflammation steadily increased and
oral hygiene was poor and the fact that Gil began to decrease and OHI began to improve only after G32 application was started, which definitely indicates the therapeutic usefulness of G32 during orthodontic treatment. This study has shown that hypertrophic gingiva produced during the orthodontic corrective treatment with appliances can successfully be treated with starting G32 application simultaneously with orthodontic treatment. The patients has complete relief from gingivitis and they showed improved Oral
Hygiene. They regained the normal gingival stippling. In addition the patients had a feeling of freshness in the mouth and felt that gums had become tougher.
G32 application can be effectively used as an adjuvant to improve tissue tone and to bring about gingival shrinkage during orthodontic treatment. It improves tissue tone and texture and also restores and maintains the gingival health during orthodontic treatment.
Toxic or Side effects
G32 is non-toxic, non-abrasive and a safe adjuvant in oral physiotherapy.
Summary and Discussions
- G32 application and massage can be
beneficially used starting with orthodontic treatment in cases of malocclusion.
- G32 application reduces Gingival Inflammation and improves Oral
- It restores the normal stippling and texture.
- Gums become tougher.
- There is freshness in the mouth.
- It has no local or systemic side
- As orthodontic treatment for rralocclusion is always prone to gingivitis, G32 application decreases gingival inflammation and brings about shrinkage during orthodontic treatment so that total course of orthodontic treatment is shortened.
Acknowledgement
Our thanks to Alarsin Pharmaceuticals, Bombay-400 023 for their cooperation.
References
Available with the Authors.